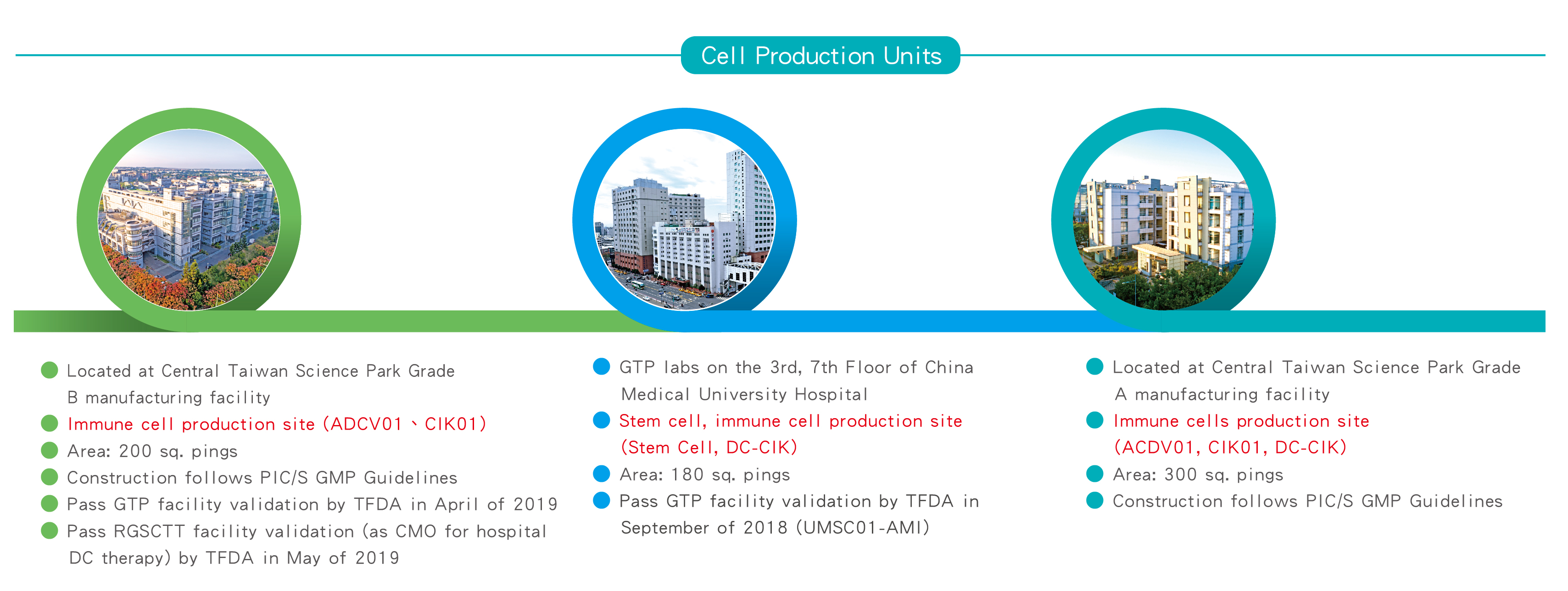
Ever Surpeme was approved by the Ministry of Science and Technology to enter the Central Taichung Science Park in February 2017. The Central Science Industrial Park is administered under the Ministry of Science and Technology, and was purposely established in 2003 to meet the needs of Taiwan’s industry in the perspective future by providing a highly excellent environment for high-tech industries and encouraging manufacturers on developing highly advanced products, and promote upgrading of the technology industry in Taiwan. Being able to receive the approval of the Ministry of Science and Technology to enter the CTSP, representing that the strength and capacity of Ever Supreme has been recognized and supported by the government.
For the purpose of assuring the manufacturing quality of drugs and prevent possible cross-contamination, misuse of improper raw materials and over use of the materials, the Taiwan FDA has required the pharmaceutical industry to fully comply with the PIC/S GMP standard on January 1, 2015 on its management system and audit. Such regulations are the latest and the most complete manufacturing requirements imposed on the pharmaceutical and biotechnology industries so far.